Elephant Toothpaste Showdown 🦷✨
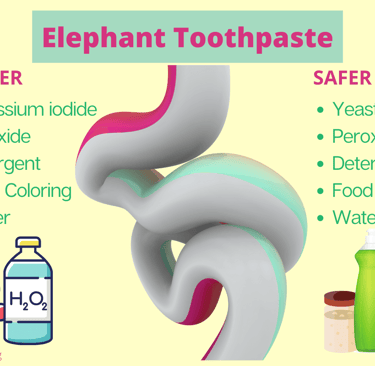
Hey scientists! Welcome back to Science with Violet! Today, we’re testing out a foamy, fizzy, colorful experiment called Elephant Toothpaste. Don’t worry—no elephants needed 🐘—it just makes so much foam that it looks like toothpaste big enough for an elephant! This experiment is all about chemical reactions. That’s when two (or more) ingredients mix and transform into something totally new. Let’s see which reaction makes the best foamy explosion. What You’ll Need: 12% hydrogen peroxide (⚠️ adult supervision required—this is a strong chemical, not the kind from the regular store!) Dry yeast Potassium iodide (optional, if you want to compare reactions) Warm water Liquid dish soap Food coloring Safety goggles & gloves 👓🧤 (safety first!) A vase or tall container Step 1: Safety First Hydrogen peroxide can sting your skin, so put on goggles and gloves before you start. Violet and her dad made sure to gear up before touching any chemicals. Step 2: Mix the Ingredients In one container, mix 1 tablespoon of yeast with 3 tablespoons of warm water. Stir it up until it looks like sandy water. In another container, you’ll later mix potassium iodide with warm water. This way, we can compare both reactions. In your tall vase, pour in ½ cup of hydrogen peroxide (12%). Add a squirt of dish soap. Add 3 drops of food coloring—Violet chose red and yellow for fun colors! Step 3: Let’s Make It Foam! First, pour in your yeast mixture. Stand back and watch it fizz and grow! Next, try the potassium iodide mixture and compare which makes the biggest reaction. The Results: The Big Showdown 🥊 Yeast mixture: Fast, foamy, and super colorful—it grew right away! Potassium iodide mixture: Slower to start, but still made a good amount of foam. And the winner is… YEAST! 🎉 It made the tallest, quickest foam explosion, while the potassium iodide was more of a slow build. The Science Behind It 🔬 Hydrogen peroxide naturally breaks down into water (H₂O) and oxygen gas (O₂). Normally, this happens slowly—but when you add a catalyst (like yeast or potassium iodide), it speeds up the reaction. The soap traps the oxygen bubbles, creating lots of thick, foamy toothpaste! Yeast contains an enzyme called catalase, which speeds up the breakdown quickly. Potassium iodide also speeds it up, but in a different way—sometimes slower. Final Thoughts This was such a fun experiment because we turned it into a science competition! Sometimes reactions are fast, sometimes they’re slow—but either way, they’re awesome to watch. 💡 Pro tip: Try adding different food colors to make rainbow foam! Thanks for watching and reading Science with Violet! If you want to see more cool experiments, head to scienceofviolet.com. Bye, scientists! 👋✨
11/23/20241 min read

Fun science adventures
